 |
Concentration of Deferoxamine in (µg/mL)
|
|
Abstract
Iron chelation therapy is used in human patients with transfusion iron overload. Deferiprone and deferoxamine are the effective chelating agents used for the treatment of iron overload in ß-thalassemic patients. As an essential trace element, iron is an important component of many proteins that play a key role in the metabolic pathways of living organisms. There is no physiological route for the rapid excretion of excessive iron. Excessive iron is toxic. These drugs also bind with other metals ions and deficiency of these in human may be critical, therefore, it is important to study the binding capacities of all these metals. Metabolism is an important factor influencing the iron-scavenging efficacy of iron chelators. Metabolism play a role in determining the boundary between safety and toxicity. The present project is designed to study the chelation of deferiprone and deferoxamine with different metals both in vitro and in vivo. Blood and urine samples will be collected from thalassemia patients for in vitro/in vivo study. Healthy human beings (24 male and 24 female) of almost same age and weight will be given the therapeutic dose for in vivo study. Blood and urine samples of each volunteer will be collected at different predetermined time intervals till 48 hours. The concentration of deferiprone, deferoxamine and their metabolites will be determined by high performance liquid chromatographic (HPLC) method and metal concentration will be determined by atomic absorption spectrophotometer (AAS).
Problem statement
Iron accumulation is an inevitable consequence of chronic blood transfusions and results in serious complications in the absence of chelation treatment to remove excess iron. There is massive outpouring of iron in blood in poorly chelated thalassemia major patients overwhelming the iron carrying capacity of transferring, resulting in the emergence of toxic non-transferrin bound iron (NTBI). Beside the chelation of iron, these drugs can also chelate other essential metals such as zinc or calcium. Chelation of these drugs with metals other than iron is also important as the deficiency of any metal can be crucial like zinc deficiency might lead to an exacerbation of the inability of the pancreas to secrete sufficient amounts of insulin in response to glucose stimulation in ß-thalassemia patients. Urinary iron excretion (UIE) in response to an iron chelator may be very useful in monitoring the iron chelation efficacy. The project is designed to study the competition for iron between the leading oral iron chelator deferiprone and deferoxamine, and the effect on other metals. The chelation of deferiprone and deferoxamine with metals present in body other than iron may be crucial. Metabolism of these chelating agents may be an important factor influencing the iron-scavenging efficacy of iron chelators.
Objectives
1.Compare the binding of deferiprone and deferoxamine with different metals both in vitro and in vivo.
2.Quantitatively evaluate iron and other metals excretion induced by the drugs. Urinary excretion of deferiprone, deferoxamine and their metabolites. Understanding of the mechanism involved in iron excretion and their overall effects on body iron levels can facilitate the improved therapeutic protocols for the treatment of conditions of iron and other metabolic imbalance and toxicity.
3. These findings may have a bearing in the design and use of new therapeutic chelation protocols to avoid metal toxicity in different metals overload patients.
Methodology
The study will be conducted both in vitro and in vivo in thalaseemic patients and normal individuals with good practice guidelines. Volunteers enrolled for this study will first be apprised in detail with all aspects of the study in easy understandable language and terminologies. Only those who will agree voluntarily and provide written “Informed consent” will be registered. Demographic data that include body weight, age, body temperature and blood pressure will be recorded. Blood and urine samples will be collected from thalassemia patients for in vitro/and in vivo study. Healthy human beings (24 male and 24 female) of almost same age and weight will be given the therapeutic dose for in vivo study. Blood and urine samples of each volunteer will be collected at different predetermined time intervals till 48 hours. The concentration of deferiprone, deferoxamine and their metabolites will be determined by high performance liquid chromatographic (HPLC) method. The metals concentration was determined by atomic absorption spectrophotometer (AAS). The data will be analyzed by regression and correlation, the result will be given as average ± SE (Steel et al., 1997). Purchase of equipment, chemicals and other supplies, Validation of analytical methods and In vitro studies of metal binding was done in first year. Collection of blood and urine samples of thalassemic patients and healthy volunteers, HPLC and atomic absorption spectrophotometric analysis of biological samples and write up of report and submission in the second year.
Results and Discussion
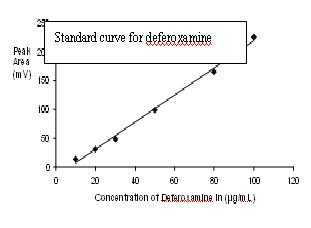
Analytical procedures for drugs ( deferiprone and defroxamine ) were developed by HPLC and standard curves for both drugs were constructed. Concentration of metal ions ( iron and zinc) in urine and plasma were determinated by atomic absorption spectrophotometer (AAS) and standard curves for both metal ions were constructed . The biochemical parameters (serum urea and creatinine, totalcholestrol, triglyceroids, High Density Lipoproteins(HDL), Low Density Lipoproteins(LDL), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), total protein, (albumin and globulin), ferritin and complete blood picture was determined. Hematological parameters white blood cells (WBC), red blood cells (RBC), hemoglobin (HB), HTC, MCV, HTC, MCHC and PLT in thalassemic patients was also studied. Most of the parameters showed elevated values of ferritin and it may be due to excessive absorption , parentral iron therapy and repeated transfusion. The hematological parameters RDW and MCV were significant MCH result showed high degree of significance. Other parameters as RBC, Hb, Hct,PLT showed non significant result. The urinary excretion of iron in male and female volunteers after deferoxamine administration was found in urine of female and male within 24 hours was 6.29 ± 3.28 and 4.99 ± 3.31 mg/ 1000 mL, respectively. After twenty-four hours of drug administration, the total zinc excreted in urine was significantly higher than without drug administration.
Conclusions
The total iron excreted in urine of female and male within twenty four hours was 6.29 ± 3.28 and 4.99 ± 3.31 mg/ 1000 mL, respectively. After twenty-four hours of drug administration, the total zinc excreted in urine was significantly higher than without drug administration . Deferiprone and deferoxamine can bind with zinc. |